Menopause and Brain Health: Misleading Advice from Oprah
And compounded estriol is NOT recommended for protecting your brain in menopause
The Oprah menopause special devoted some time to brain health, and sure enough, I’ve already had questions about it as many people took away that every woman needs to be on estrogen for brain health…and STAT!
After watching the segment, I can absolutely understand why women were frightened and why it would lead them to think they needed to immediately start menopause hormone therapy (MHT) to protect their brains.
I admit I was surprised to hear Dr. Rhonda Voskuhl, a Professor of Neurology at the University of California Los Angeles (UCLA) and faculty neurologist for the UCLA Comprehensive Menopause Care Program, imply that menopause is a “neurodegenerative disease” or at least is like one. We were also told that women need to start treatment after the “first hot flash.” I was honestly stunned.
She also said that “these cognitive changes and structural changes in the brain don’t suddenly get better,” and “I do think it needs a drug,” specifically hormone therapy.
In contrast, here is a summary of the Study of Women’s Health Across the Nation (SWAN) on cognitive changes1 :
SWAN’s initial, 4-year longitudinal analysis of the relation between the MT and cognitive performance disclosed a temporary decrement in both processing speed and verbal episodic memory during the perimenopause; the decrement resolved in postmenopause. The negative perimenopause effect was subtle, manifest as the absence of a learning effect, meaning that cognitive test scores did not get better with repeated administrations. Improvement with repeated testing is normative in this age range. But even in perimenopause, test scores did not drop—they simply did not improve during this MT stage.
(MT=menopause transition)
There was an in depth update on brain health and menopause at the 2024 Menopause Society meeting, which I wrote about here. It was given by Dr. Pauline Maki PhD, a leading neuroscientist who is active in menopause research and whose work I respect very much. She told us that the 2022 Menopause Society guidelines hold, and then she laid out an expertly crafted review of the literature to support the Society’s position that menopause hormone therapy (MHT) is NOT Indicated for cognition or to prevent dementia. She said that people telling women that estrogen therapy is essential to prevent dementia are operating in a “data-free zone.” 2
There are lots of hypotheses about menopause, brain health, and dementia. The best we can say is that women with more hot flashes appear to be in the higher risk group, but we don’t yet know if this is causal. One possibility is that the hot flashes are negatively affecting the brain, but another possibility is that the same thing that causes women to have hot flashes is the same thing that raises their risk of dementia, meaning it is correlation. But even if it’s cause and effect with hot flashes, we need studies to show that a medication makes a difference. Also, if a medication makes a difference, is it estrogen or any treatment for hot flashes? After all, there is a study that treating hot flashes with a nerve block, so no estrogen, improves memory.
I don’t know of any study with any estrogen to treat brain fog, and, as we heard at the Menopause Society meeting in 2024, there is no evidence yet to support prescribing MHT for the sole purpose of preventing dementia. I thought, might I have missed something? Sometimes when I do searches for some reason or another a paper gets missed, so I asked Dr. Maki some questions by e-mail and she confirmed, “Not only are there no randomized trials of estriol for "brain fog" in peri menopausal or postmenopausal women, there are no trials supporting the use of any estrogen for "brain fog".
MHT has been studied for cognition in women in early postmenopause. There are four randomized controlled trials with up to seven years of follow-up. None of them showed a benefit for cognition. Here is the relevant slide detailing the four studies from Dr. Maki’s talk.
We were also shown this slide, which shows that several observational studies have linked MHT with an increased risk of dementia.
This does not mean that people should freak out if they are taking MHT; it means that we do not yet have the answers. And so, while science is trying to sort out this complex issue, we should be offering treatment to people who are suffering from hot flashes so they do not suffer. If it turns out that these women are most likely to benefit brain-wise from therapy, well, they have been treated!
Back to Oprah.
What is noteworthy about Oprah’s menopause special is what we were not told–that Dr. Voskuhl is the inventor of a therapy that women supposedly need for their degenerating brains.
This conflict of interest might not matter to Oprah, but it matters to me.
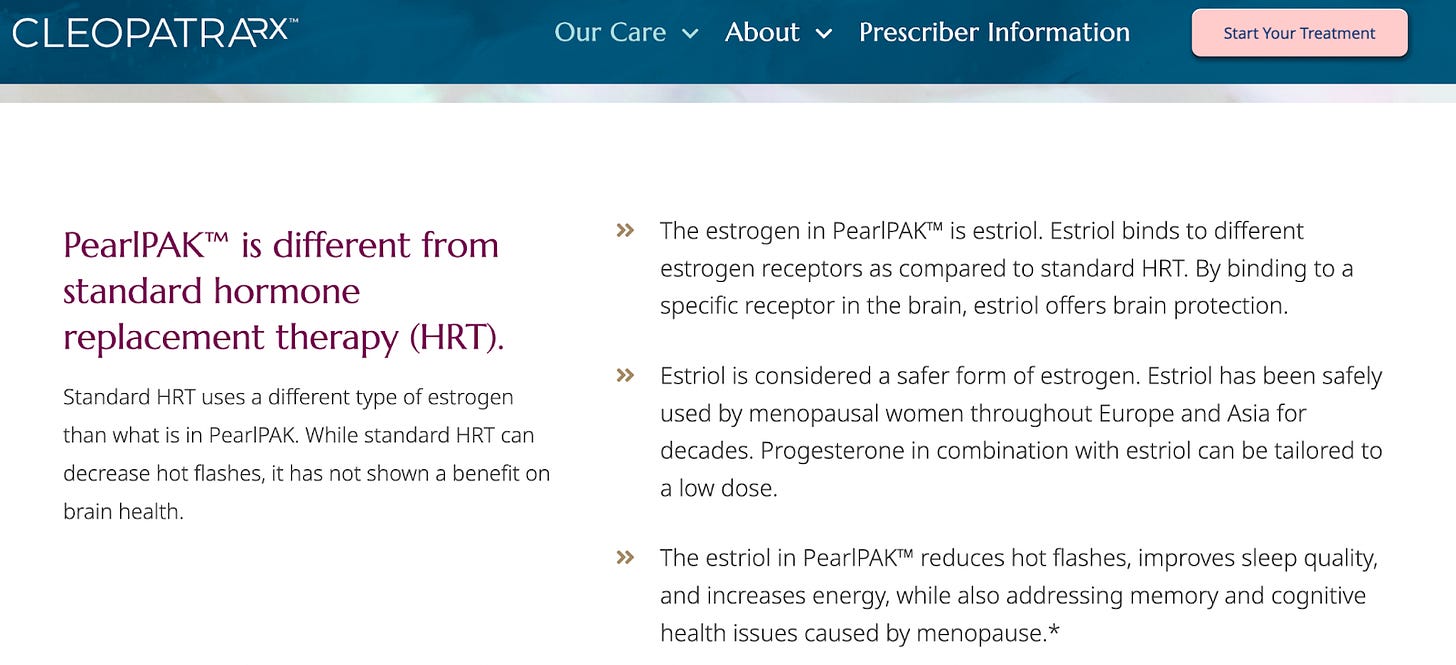
I wondered what claims I might find about PearlPAK™ over at CleopatraRx:
Reading this, a reasonable person could not be faulted for thinking PearlPAK™ is supported by robust randomized-controlled clinical trials in perimenopause and menopause. The therapy claims to be “clinically tested and validated,” which might even lead some people to think that the product is FDA-approved, when in reality, that is marketing terminology and, in my opinion, the equivalent of saying, “My mom likes it.”
And what is PearlPAK™? Compounded estriol (with progesterone if you also need that). If you have been reading this Substack for a while, you know that all guidelines advise against compounded hormones as first-line treatment.
Okay, let’s unpack (unPAK?) it.
What is Estriol?
Estriol is one of the four main naturally occurring estrogens:
E1 or estrone, made by the ovary and other tissues, does not decrease much during menopause and levels may even stay the same.
E2 or estradiol, made by the ovary and other tissues, decreases significantly in menopause.
E3 or estriol, made chiefly by the placenta; small amounts are also produced during the metabolism (breakdown) of estrone.
E4 or estetrol, made by the fetal liver.
Is Estriol Special or Novel?
Estriol isn’t popular for MHT because it doesn’t bind as well to the estrogen receptors as estradiol (something needed for the hormone to have an effect), and it is also cleared more quickly from the body. These properties affect its usefulness and typically means it needs to be taken twice a day to be helpful for symptoms. It’s fair to think of estriol as a weaker estrogen. Interestingly, it does have a better binding ability in vaginal tissues, so it is effective, just like estradiol, when given vaginally for genitourinary syndrome of menopause.3
There is a misconception that because estriol is a weaker estrogen for most tissues, it may be safer. However, like estradiol and Premarin, it can stimulate the endometrium and raise the risk of endometrial cancer. 3-5 Lots of people like to talk up the mechanistic effects of hormones when discussing safety. For example, estriol binds in this way, or estradiol binds that way to a receptor, but to support the claim that estriol is safer than estradiol or any other estrogen, we need safety studies in real, live human women where estriol is compared with estradiol.
Estriol has been studied for hot flashes, but in randomized placebo-controlled trials, doses up to 4 mg were less effective than 0.625 mg of Premarin and 2 mg of estradiol valerate.6 Oral doses as high as 6-8 mg a day might be needed, and apparently 8 mg is what produces the same estriol levels as pregnancy. Whether it is safe to be exposed to estriol levels that we would experience during pregnancy for years or even decades beyond the age when pregnancy can occur is simply unknown. Also, I was left wondering how many of the uncomfortable symptoms of pregnancy are due to high levels of estriol?
There have been claims that estriol is safer for the breast, but I can’t find any robust clinical studies to back up that assertion. I asked Professor Sue Davis, an endocrinologist and a world-renowned expert in reproductive hormones, about estriol, and she told me via e-mail, “It has never really had much popularity because it has not been shown to be as effective as other forms of MHT. To my knowledge, breast cancer data are lacking.”
The long and short of it is that estriol was primarily abandoned because it seemed less effective, more cumbersome, and no safer. As it has been studied less, we have much less data to evaluate safety.
People Have Been Trying to Make Estriol Happen for Years.
Think of this in your best Regina George voice from Mean Girls, “Stop trying to make fetch happen,” except replace fetch with estriol.
There is no FDA-approved version of estriol in the United States. In Canada there is a vaginal estriol product approved for genitourinary syndrome of menopause (GSM), but to my knowledge no oral or transdermal formulation of estriol is approved by Health Canada. In the United Kingdom, there are several vaginal estriol products, but no approved oral or transdermal preparation according to the EMC (electronic medicines compendium).
Estriol has appeared in various compounded hormone preparations in the United States and other countries; many people may be familiar with the unregulated and not recommended compounded products known as Bi-Est (compounded estriol and estradiol) and Tri-Est (compounded estrone, estradiol, and estriol). These compounded hormones were circulating in the alternative medicine world as far back as the 1980s. They were catapulted into the mainstream when Suzanne Somers started writing about and promoting her completely unstudied and unsafe hormone regimens. Oprah gave these unstudied and not recommended therapies a mega boost in 2009 with her menopause special. You can read more about Somers and Oprah’s contribution to menopause misinformation here.
The Claims About Estriol Today
This is from the CleopatraRx Website:
It is correct that “Standard HRT” (and, infuriatingly, they use the old, misogynistic HRT instead of the accurate MHT) “has not shown a benefit on brain health.” But then there is mention of receptor binding and estriol being safer (which I’ve already addressed) and a claim that “estriol offers brain protection” and addresses “memory and cognitive health issues caused by menopause.” Anyone reading this could not be faulted for thinking that PearlPAK™ has been studied and shown to be effective for brain health in perimenopause and menopause and that it succeeds where other estradiol or Premarin-based MHT fails.
I headed over to their “Research” section, wondering what they were offering as proof of efficacy and safety–it is…disappointing.
Seven papers are listed on their website as of April 6, 2025.
Four are mouse studies, and, well, women are not mice. Also, three of the studies are mice models to study multiple sclerosis (MS), which is not menopause. I am not saying this PRE-CLINICAL work is not valuable, but we don’t jump from a mouse study to prescribing medication to women. These papers are not relevant to prescribing a drug to women for perimenopause and menopause.
There is a fascinating review article that I thoroughly enjoyed, but it largely discusses preclinical work and concludes that “Such preclinical findings can serve as a basis for translation to clinical trials in humans. This will inform whether sex hormones, sex chromosomes, or both contribute to sex differences in neurodegeneration across the lifespan.” This paper does not conclude that estriol protects the perimenopausal and menopausal brain.7
There are two phase two studies…of women with multiple sclerosis (MS) receiving a dose of 8 mg of estriol a day, which is meant to mimic the levels during pregnancy. This simply isn’t applicable for menopause.
The website does not list one clinical trial in women that supports oral estriol to address “memory and cognitive health issues caused by menopause.”
I thought, might I have missed something? So I asked Dr. Maki by e-mail, and she confirmed, “Not only are there no randomized trials of estriol for "brain fog" in peri menopausal or postmenopausal women, there are no trials supporting the use of any estrogen for "brain fog."
PearlPAK™ is Just Another Non-FDA Approved Compounded Hormone
The estriol in PearlPAK™ is yet another compounded product. It seems there is an option to get an FDA-approved progesterone.
It’s disappointing but not surprising to see the same marketing language from CleopatraRx, a “natural solution” and an “exclusive blend,” that could just as easily have come from Suzanne Somers.
Also, compounded estriol is not a natural product by biochemical definitions, it is typically made by semi-synthesis.
And mind the gap? What about the gap in clinical trial data on women in menopause supporting this product?
I was shocked by the prescribing information. Am I supposed to start someone on 2 mg of estriol, the “standard prescription,” or should I blast them with the pregnancy dose of 8 mg? What do the studies say is best, and how long does it take to see an effect on the brain?
Right. We need studies.
The progesterone information feels very cavalier to me. How much progesterone is needed to protect the uterus from 6 or 8 mg of estriol? The two studies listed on the CleopatraRX website that used 8 mg of estriol for MS did not give progesterone, and there was no endometrial safety data. Also, the women were mostly not in menopause. Based on the information provided, I have no idea how to dose progesterone with these doses of estriol.
But at least I can get a personalized prescription pad!
Don’t Trust Oprah on Health
Some people will say, ”But at least Oprah got the conversation started; women are desperate for information.”
Yes, they are, which is all the more reason to get it 100% correct because we are all more inclined to believe the first piece of information we hear.
Given the catastrophic dismantling of the FDA, CDC, and NIH, my prediction for 2025 and beyond is that we can expect to see more companies creating bespoke compounded hormones for menopause, claiming they are tailored for this symptom or that. There is simply too much money to be made. We’ve already seen Hims & Hers brazen and misleading Super Bowl advertisement about their compounded weight loss medications.
In the United States, compounding creates all kinds of loopholes for promoting products with hyped up claims while ignoring side effects and complications. Now that the FDA is crumbling, is anyone even around to send anemic warning letters? Is there still a team for safety inspections of compounding pharmacies? The number of them with safety violations that put consumers at risk will shock you.
While menopause hormone therapy absolutely has its place in managing certain symptoms, the sweeping claims that estrogen is the key to treating brain fog or preventing cognitive decline or dementia are not backed by solid scientific evidence. In my opinion, women deserve better than inadequately studied compounded estriol offered as a miracle cure.
And so I was right; you can’t trust Oprah to handle menopause correctly.
References
El Khoudary, Greendale G, Crawford SL, et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause 2019; 26:1213-1227.
Maki PM, Jaff NG. Brain fog in menopause: a health-care professional’s guide for decision-making and counseling on cognition. Climacteric 2022;25:6,570-578, DOI: 10.1080/13697137.2022.2122792.
Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005; 8:sup1, 3-63, DOI: 10.1080/13697130500148875.
Weiderpass E, Baron JA, Adami HO, et al. Low-potency oestrogen and risk of endometrial cancer: a case-control study. Lancet. 1999 May 29;353(9167):1824-8. doi: 10.1016/S0140-6736(98)10233-7. PMID: 10359406.
Scialli A, Fugh-Berman A. Maturitas 2003;45:47.
Volpe A, Facchinetti F, Grasso A, et al. Benefits and risks of different hormonal replacement therapies in post-menopausal women. Maturitas 1986;8:327–34
Voskuhl R, Itoh Y. J. Exp. Med. 2022 Vol. 219 No. 12.











All your articles are so vitally important. It's so easy to be duped. A dear friend of mine, who is incredibly intelligent, said that I should listen to the recent menopause episode of the Armchair Expert because there was a menopause expert featured. My first question was "is she selling something?" And my friend replied, "her name is Dr Mary Claire Haver, and she is selling something, but it's because she realized that women's needs aren't being met." I nearly fell over and immediately started sharing your newsletter. Point being, I wish you were as famous as Oprah, and will do my little part to make this so.
Another excellent analysis. When I first heard about the recommendation for estriol for brain health, my immediate impulse was to pull up the slides from Dr. Maki's lecture, which, as you point out, substantiated that these claims are ...unsubstantiated. Then, I called Dr. Maki to make sure there wasn't any new data that I had missed. No new data. Just a new person fearmongering to make money.