Fezolinetant, the new drug for hot flashes, gets approved by the FDA
A deep dive into what you need to know
One open secret in medicine is that many supposedly “new” drugs aren’t really new at all, they are simply a newer version of a drug that had already been approved. These are known as “me-too drugs,” and while the modification may produce a statistically significant difference versus the older drug and this is how they get approved by the Food and Drug Administration (FDA), these differences are rarely meaningful to the people who need the medication. Typically, the only major difference between the older drug and the newer “me-too” version is the price. “Me-too” drugs are simply a way to get more money out of a drug once the patent is about to expire.
I hope this helps everyone understand the excitement of having a genuinely new drug, entirely different to anything we’ve seen before, especially one that is for menopause!
The Medication
The generic name is fezolinetant and the trade name is Veozah, and it is for hot flashes in menopause. It is not a hormone, rather it is in a class of drugs known as neurokinin-3 receptor antagonists (meaning it blocks that receptor). I admit that’s a mouthful, but we’re going to break it down. The first step is to understand a little more about what is happening in the brain during a hot flash.
The Neurobiology of a Hot Flash
Body temperature and reproduction are linked. This is why temperature rises a little during the luteal phase of the reproductive cycle. Part of this interrelated signaling is controlled by KNDy neurons (pronounced candy), which send signals about heat to the thermoregulatory center in the brain (this isn’t all the KNDy neurons do, just an FYI). KNDy neurons send their signaling via a chemical called neurokinin B, but neurokinin B also further stimulates the KNDy neurons. Neurokinin B must interact with the neurokinin 3 receptor to work. Estrogen suppresses the KNDy neurons.
Think of neurokinin B as turning the temperature dial up and estrogen as turning it down. The thermoregulatory center also takes in input from the environment and then makes decisions, as it were, about temperature control. Check out my handy infographic below (and no, I will clearly not be giving up my day job for graphic design).
KNDy neurons lose the counterbalance to their messaging about heat when estrogen levels decrease. In addition, without estrogen, the KNDy neurons enlarge, so they can send more heat signals. See below.
A sensation doesn’t happen until the brain says it happens, meaning you do not feel heat, even if you have your hand in an open flame, unless your brain assembles the chemical signal that it receives from your hand as heat. With a hot flash, you aren’t feeling hot because your body temperature is rising, what is happening is that you are receiving an incorrect chemical signal that it is! Basically, the call is coming from inside the house. Meaning, your brain has assembled a message of excess heat because it received a signal from the KNDy neurons, and now as far as your brain is concerned (which is all that matters), you are hot and so you feel hot.
(Hot flashes are biologically very complex, and there are likely other mechanisms involved, but we’re keeping it to the confines of how fezolinetant works).
Lots of people wonder how it is possible that your body temperature hasn’t risen, even though your skin is very hot to touch (for me, I can be hot enough to wake my partner sleeping next to me!). Skin is hot with hot flash because the brain, mistakenly thinking you are hot, starts to deploy the mechanisms to cool down. This involves dilating blood vessels and shunting blood to the skin so you can dump body heat from blood. This is also why many people sweat during a hot flush. Because core temperature was never elevated, body temperature can actually drop after a hot flash because the body has deployed mechanisms to cool off. This is why some people feel cold and shiver after a hot flash.
How Fezolinetant Works
Fezolinetant blocks the neurokinin 3 receptor, which is the receptor that neurokinin B uses to communicate. Basically, fewer heat messages get through and the stimulation of the KNDy neurons also decreases, so production of neurokinin 3 also decreases.
Fezolinetant restores some of the temperature balance in the brain that was lost because of the decrease in estrogen.
How Well Does it Work?
Fezolinetant has been studied in several placebo-controlled trials. Two of the studies used for FDA approval were placebo-controlled for the first 12 weeks, and then at 12 weeks the women who received placebo were given fezolinetant for the remaining 40 weeks. A third study was randomized and placebo-controlled for the full 52 weeks and this was a safety study.
A bit about the women who were enrolled in these studies:
Ages 40-65. This encompasses the time that hot flashes from menopause are most expected. If periods stop before age 40 it’s primary ovarian insufficiency and biologically that isn’t quite the same as menopause.
17% of participants were African American. This is important as often medications are understudied for African American women.
Women had to have at least seven moderate to severe hot flashes a day to be in the study and they used a mild, moderate and severe grading of hot flashes.
Some women had previous hysterectomies, some had their ovaries out, and some had previously been on hormone replacement therapy (but there was a wash out so the effect would truly be from fezolinetant and not the estrogen). This is good because it reflects the range of menopause experiences.
The BMI range for inclusion was 18-38.
Women had to be in menopause, meaning no one was still having irregular periods.
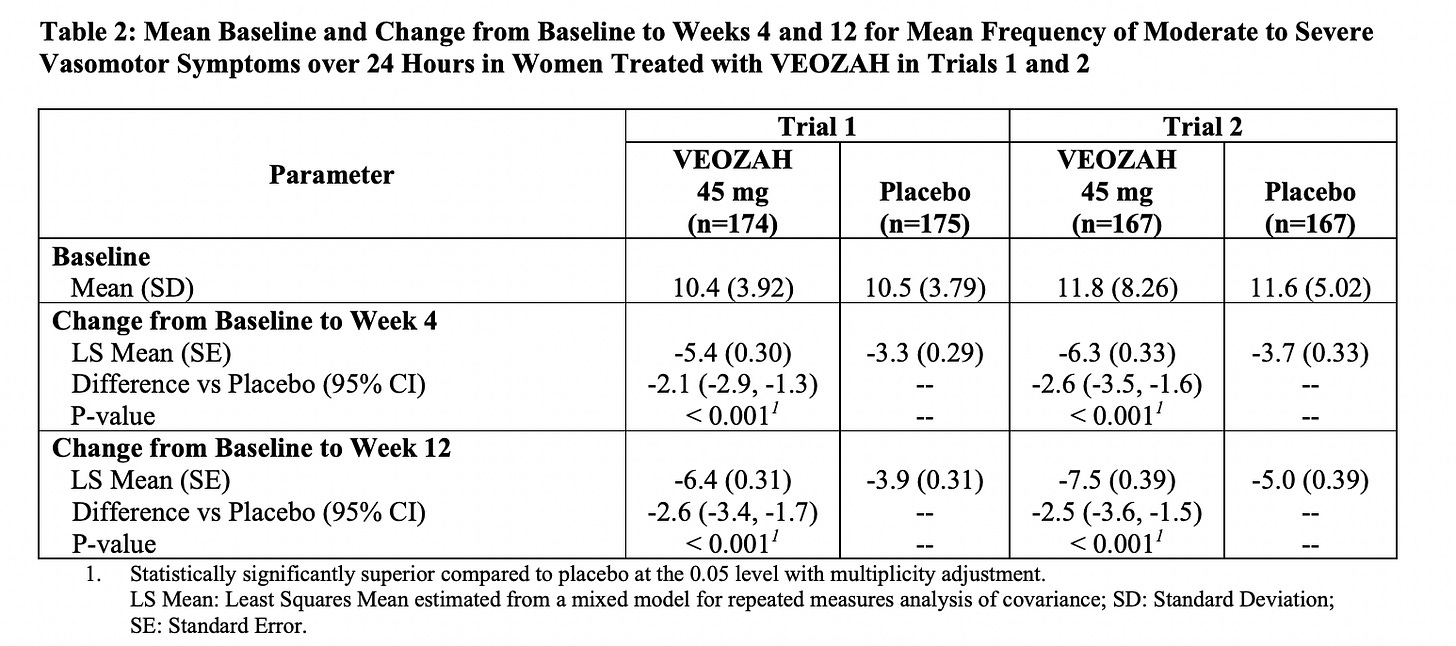
The average number of hot flashes a day before the drug was 10.4 for study 1 and 11.8 for study 2. By 12 weeks the average went from 10.4 a day to 4 a day in study 1 and 11.8 a day to 4.1 a day in study 2. It’s also encouraging to see that there was a significant effect by week 4 and some women actually responded as early at the first week.
The table below is from the product monograph, and I’ve included it because it’s a good way to see the powerful impact of placebo. Now think about every supplement that promises it can help hot flashes, but doesn’t have a placebo controlled study to provide a comparison.
Here’s another way to look at how well the drug worked. Overall, about 58.7% of people who took fezolinetant had a 50% reduction in hot flashes versus 36% for placebo and 37% had a 75% reduction in hot flashes versus 17% for placebo.
As for the severity of hot flashes, this was scored based on mild, moderate and severe, which was a scale of 1-3 in the studies. The average severity score was 2.4 before the medication and it reduced by 0.6-0.8 with fezolinetant. This was also a greater improvement than placebo. This is kind of a weird way for doctors to communicate with people about their hot flashes. The bottom line is that quality of life metrics, including sleep scores, were better with fezolinetant than placebo and that the intensity of hot flashes decreased for some.
There is always a big caveat with how well a drug works when all we have are Pharma funded studies. As the drug is prescribed in the real world and we get more data, we will learn more.
How Does Fezolinetant Compare with Estrogen and Other Options?
Fezolinetant has not been studied head-to-head with estrogen, so we can’t say for sure how it compares. However, we do know that a 50 mcg estradiol patch or 1 mg pill of estradiol (these are equivalent doses) will improve hot flashes for about 80% of people, so it would seem that overall estrogen performs better. However, many people can’t take estrogen or have side effects from it, so a gold standard is of no use to people who can’t take it. And of course there are the 20% of people who don’t get much relief from estrogen and who may do better with a different approach.
The bottom line here is that options are good.
Side Effects and Risks
Some people will make a stink over the fact that 43% of people taking fezolinetant reported adverse events, but the rate for those taking placebo was 45%. Again, this is why it’s so important to have a placebo.
The chart from the product monograph abstracts the significant side effects/risks and their relationship to placebo. Yes, 2.5% of people taking the drug reported hot flashes from the medication, but so did 1.6% who took placebo.
Side effects to watch out for that are more likely to occur than placebo, but are still uncommon, and are abdominal pain, diarrhea, and insomnia.
Let’s talk about the liver as 2.3% of people taking fezolinetant had elevated liver enzymes, meaning there was a negative effect on the liver. This also means that for 97.7% of people there was no effect on the liver. These changes reverted to normal when the drug was stopped, so there was no permanent negative effect. This is something that can be seen with a variety of medications, and shouldn’t freak people out. What it means is checking liver enzymes is part of prescribing, as it is with some other drugs. Liver tests should be done before starting and at 3, 6, and 9 months into therapy. People with liver disease shouldn’t take fezolinetant and neither should people with kidney disease.
To put these liver changes into perspective, light to moderate alcohol drinking is associated with an elevation of liver enzymes and it appears that this risk is higher for women who are 40 years and older. In fact, in one study a woman aged 40 and older was at increased risk of elevation of one of the liver enzymes if she had just 4 drinks a week.
Drug Interactions
Fezolinetant is metabolized by the enzyme CYP1A2, so should be avoided for people taking medications that inhibit that enzyme, otherwise levels of fezolinetant can rise. Common medications that might be of concern here are:
Cimetidine, trade name Tagamet, used for heartburn.
Ciprofloxacin, or Cipro, an antibiotic
Ethinyl estradiol, which is the estrogen in many birth control pills and in a few formulations of menopausal hormone therapy.
Fluvoxamine (Luvox, and antidepressant also used for obsessive compulsive disorder and anxiety disorders)
Cost
According to the New York Times, the company that makes fezolinetant, Astellas, will be charging $550 for a 30 day supply, or a whopping $18.33 a pill/day. The Institute for Clinical and Economical Review (ICER) felt the drug would be cost-effective for people at $5.48-6.84 a day (the ICER considered factors such as how well the drug works and the financial impact of hot flashes, both from seeking medical care and on missing work, among other things). If the ICER is correct, a lot of people won’t think the drug is worth it at $18.33 a day.
Developing a drug is expensive, but Pharma also rakes in billions so clearly the drugs are overpriced. I’m not a market analyst, but I think if the Veozah were priced lower, more people would likely try it and the company could make the same amount by selling more product. What is a particularly bothersome point for me is the primary market is likely women with breast cancer who can’t take estrogen, and in the United States these women are already paying a lot more for their healthcare.
But is Veozah uniquely obscene price-wise, meaning women are being taken advantage of disproportionately, or obscene within the normal bounds of obscenity for American pharmaceuticals?
When Viagra came out in 1988 it was about $10 a pill. Adjusted for inflation, that is about $18.61 a pill today…which coincidentally (or not) is almost exactly the price of Veozah. I don’t really believe in these kinds of coincidences, so my guess is the Viagra experiment taught the pharmaceutical industry that the American market will bear $18 a day for a drug that is related to quality of life for reproductive health.
Sigh.
Generic estrogen patches are $50.84 a month and generic oral estrogen is $5.10 a month via Cost Plus Drugs, so $550 a month for Veozah is just not going to be a viable alternative for most people who have hot flashes and who can take estrogen. Yes, some insurers will bear the cost, but that just adds to the overall cost of health insurance.
Exciting New Things
Fezolinetant has been studied in higher doses for polycystic ovarian syndrome, and interestingly it helped reduce some of the abnormal hormone levels seen with that condition. I think we can expect more research here.
What is the Take Away?
Fezolinetant, 45 mg a day, looks promising. It doesn’t appear to be as effective as estrogen for hot flashes, but it seems to be effective enough.
The pricing is ridiculous in that uniquely American way, and so that will almost certainly limit the medication to people with insurance.
The people who might want to consider fezolinetant are those for whom estrogen is contraindicated, for example people with breast cancer, or those who can’t take or tolerate estrogen (this also includes those people with a uterus who want to take estrogen, but who can’t tolerate progesterone), and for those people who have tried estrogen but it doesn’t work very well for them.
We don’t have long-term or real world data (this is how it is for every new drug), so we will learn more over time.
A small percentage of people develop abnormal liver tests, so testing is required. For those who develop the changes, they are reversible.
More options are always good and it's possible we may find more uses for this medication, which is also exciting.
As with all therapies for hot flashes, nothing will work 100%. What you are looking for is a quality of life improvement.
I am looking forward to having fezolinetant as one more tool in my menopause toolkit. I just wish it weren't so expensive.
References
Modi M, Dhillo WS. Neurokinin 3 Receptor Antagonism: A Novel Treatment for Menopausal Hot Flushes. Neuroendocrinology. 2019;109: 242–248.
Rance NE, et. al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: A novel hypothesis on the mechanism of hot flashes. Front Neuroendocrinol 2013;34 doi:10.1016/j.yfrne.2013.07.003.
Angell M, Excess in the Pharmaceutical Industry. CMAJ. 2004;171: 1451–1453.
Lederman S, at. Al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet 2013;401:1091-1102.
Neal-Perry G, et. al. Safety of Fezolinetant for Vasomotor Symptoms Associated With Menopause. Obstet Gynecol. 2023;141:737-747.
Beaudoin FL, et. al. Fezolinetant for Moderate to Severe Vasomotor Symptoms Associated with Menopause: Effectiveness and Value; Evidence Report. Institute for Clinical and Economic Review, December 1, 2022. https://icer.org/wp-content/uploads/2022/06/ICER_Menopause_Evidence-Report_12012022.pdf
Niemelä O, et. al. Where should the safe limits of alcohol consumption stand in light of liver enzyme abnormalities in alcohol consumers? PLoS One. 2017; 12(12): e0188574.
Horn, JR et. al. Get to Know Enzyme CYP1A2, Pharmacy Times https://www.pharmacytimes.com/view/2007-11-8279
Product Monograph Veozah https://www.astellas.com/us/system/files/veozah_uspi.pdf









Just an added note on placebo response: When Ortho Pharm. tested Ortho Tri-Cyclen to get an indication for improving acne, it performed better than placebo, but about 36% (if my memory isn't too faulty) on the placebo also showed improvement.
Thanks for the deep dive on this, and the graphics are charmingly good. It will help me to be conversant about this with my people in primary care, and perhaps prescribe at times... but I think I will still recommend “talking to your Gyn” first. I do wish the hot flashes went to zero for that price, and very odd how the inflation adjusted cost parallels Viagra. I think brexafemme was introduced at $700/course, and the GLP 1 drugs like semaglutide run 800-1000/ month, so it’s kind of the going rate these days in America?