Hot Flashes & Hot Takes: Day 1 at the British Menopause Society Meeting
Calcium calculator, mood and perimenopause, and yes, even red light therapy for the vulva. Here's what caught my eye.
I’m at the British Menopause Society annual meeting and day one was an interesting day with talks on what’s new and breaking, depression in perimenopause and menopause, bone health, and more.
I wanted to give some quick updates from the first day of topics that that piqued my interest, either because they’re important, new to me, interesting or some combination of the three. I’ll also do the same for Day 2, and perhaps write a couple of longer posts based on some of the topics that were covered.
The University of Edinburgh Calcium Calculator And How Much Calcium Do You Really Need?
How much calcium are you really having in your diet? While it’s possible to look at all the nutritional labels and add up the milligrams of calcium, that method still doesn’t include everything that you eat, because vegetables don’t have labels and neither does restaurant food or the dinner you had at your friend’s house. And of course it’s cumbersome to search online for the calcium content for each thing that you ate.
For anyone interested in a closer look at what they might be taking in from their diet calcium-wise, there is the Edinburgh Calcium Calculator (click here) We were introduced to this in one of the lectures on bone health. (It’s probably known to the Brits, but I’d never heard about it). It’s a weekly calculator, so you still need to write down everything that you’ve eaten for the week, but this might be an easier way for some people to do a spot check on their calcium intake.
We had two lectures on bone health and we heard different recommendations for dietary calcium intake, 1200 mg and 700 mg. I’ve read a few things lately about 700 mg perhaps being enough in the diet, so this interested me. The lecturer who recommended 700 mg felt that if people were getting 700 mg a day with the Edinburgh calculator, because it doesn’t catch every source of calcium, people were almost certainly getting more than 700 mg a day.
All the recommendations are still 1,200 mg for women in menopause, but how much calcium people really need is a topic that interests me, so I’m going to take a deeper diver in a future post. I am curious how many women are taking a calcium supplement, so if you feel like answering and you are age 40, or older, please consider answering my completely non scientific poll…
Perimenopause and Depression
Dr. Claudio Soares gave a lecture, “Management of Perimenopausal Depression.” Several excellent longitudinal studies inform us that depression is 1.4 times more common in perimenopause versus premenopause. This data comes from these studies:
Harvard study mood and cycles
Penn ovarian aging study
SWAN
Australian Longitudinal Study of Women’s Health
Peking Union Medical College Aging Longitudinal Cohort of Women in Midlife
I listed the studies because I think it’s important for women to know that researchers are trying to understand more about menopause and mood. What’s really nice to report here is that we have fairly similar results from several good data sets–it’s when results can be replicated in this way that we gain much more confidence about the data and conclusions.
Dr. Soares emphasized that a prior history of depression is a major risk factor for depression in perimenopause, and previous PMS/PMDD and post partum depression as well. This is important to know so women and their providers can be prepared.
We heard that both antidepressants and estrogen are valid approaches for depression in the menopause transition, and I think that is important to emphasize this as on social media there are people demonizing doctors for prescribing anti depressants. The guidelines actually tell us that both are options to consider, and, contrary to what some people think, desvenlafaxine has been specifically studied for depression in the menopause transition. Some women will do better with an antidepressant, some with estrogen, and some with both. More options benefit more women.
One thing that wasn’t covered (and of course, one can only address so much in 45 minutes) so that some women with depression in perimenopause do very poorly with estrogen as therapy. This may be because of LOOP ovulation, which can produce cycles where estradiol levels are significantly higher than normal. Adding pharmaceutical estradiol produces even higher levels and exacerbates the wide swings in estradiol. For this reason, some women do much better with an estrogen-containing oral contraceptive, which, because it inhibits ovulation, results in stable hormone levels.
We also heard that there is a lot of work trying to untangle mood changes, hot flashes and night sweats, and sleep disturbances. There are many ways these three could be interrelated. For example, most people can imagine that chronic fragmented sleep might affect mood. Or that mood might affect tolerance for hot flashes. But it’s also possible that mood, hot flashes and night sweats, and sleep are interrelated because they share the same neurobiology.
We heard that there was hot of the press research that had just been presented only two days before at the RCOG World Congress looking at data from SWAN that may help us untangle that Gordian knot. The data is not published yet, so not peer-reviewed and I heard it second hand (from the presenter who heard it at the RCOG meeting), so all I can really say is that the work seems to really strengthen the concept of a connection between mood symptoms, hot flashes and night sweats, and sleep issues, so hopefully we’ll have that paper soon!
Incretin-based therapies and MHT: Potential Absorption Concerns with Oral Therapy
In the “late breaking” news session, we heard about several important papers and statements from the past year, and one was the British Menopause Society Tool for Clinicians on Incretin-Based Therapy and Menopause Hormone Therapy (which you can find here). The concern is incretin-based therapies (including glucagon-like peptide-1 receptor agonists or GLP-1s, such as semaglutide, and the dual glucagon-like peptide-1 and glucose-dependent insulinotropic peptide agonist, such as tirzepatide, could theoretically affect absorption of oral menopause hormone therapy. If these drugs indeed have this effect, it would be particularly concerning for women with obesity who are taking MHT if they don’t absorb their progestogens as these women are at higher risk for endometrial cancer. In addition, reduced absorption of estrogen could lead to emergence of symptoms.
The question is, do we need to be worried about the absorption and bioavailability of estrogen and progestogens and does it vary drug to drug? Unfortunately, there isn’t much data. Tirzepatide has been shown to negatively affect absorption and bioavailability of estrogen containing birth control pills, and while semaglutide should in theory have the same effect, small studies have not shown that it impacts absorption of birth control pills.
We essentially have no data here. It’s a legitimate concern that GLP-1/GIP medications could affect absorption of either or both oral estrogen and progestogens. The most concerning is the progestogen, because that may leave women at risk for endometrial cancer. The best approach is to switch to transdermal therapy for both estrogen and progestogen (like the combination patch or combination vaginal ring) or consider transdermal estradiol and a hormonal IUD for endometrial protection. We don't have good data on vaginal absorption of progesterone to protect the uterus, and while many people do it, you are really switching one unknown for another. The BMS paper states, “Where oral progestogen is preferred by patients on HRT, there are no data to inform the dose adjustment required or endometrial protection in high-risk women, including those treated with incretin-based therapies. A potential approach is to temporarily increase the dose of oral progestogen for 4 weeks after commencing incretin-based therapies, and maintain a higher dose of progestogen with each dose increment on incretin-based therapy until a stable dose is achieved. This is based on extrapolation from COC data and intuitive expert opinion. Uncertainty should be shared with the patient to aid informed decision making.”
While weight loss will ultimately lower the risk of endometrial cancer, using transdermal estrogen while absorbing insufficient progestogen because of incretin-based therapies still puts women at risk for endometrial cancer. This is especially important as these medications are generally for life, not short term use. Hopefully, as we gain more data about real world use of incretin-based therapies we will be better able to make evidence-based recommendations, but for now the combined patch, the combined ring, or IUD delivery is considered the best option for progestogens for women taking incretin-based therapies.
Update on DEXA and FRAX Scores
One of the two osteoporosis lectures provided some good basic information on assessing fracture risk. People get very worried about their T scores on their DEXA scan, but the emphasis here was that DEXA doesn’t tell us the whole story about fracture risk, and it’s fracture risk that really matters. DEXA measures approximately 50% of bone strength, so it doesn’t give us the whole picture. There were two endocrinologists who gave separate lectures, but they both told us that a woman with a barely osteopenic T score could easily have a higher fracture risk than someone with a T score of -2.5, which is the definition of osteoporosis.
Here are some bone health tidbits from the two lectures:
When looking at your DEXA scan take the lowest T score from the sites measured to assess risk.
A low T score in the lumbar spine represents a higher risk than the hip.
A FRAX score can be done with or without a bone density. With bone density, it can inform you about recommended management. Without the bone density data it can be used to calculate estimated fracture risk and if screening earlier than guidelines is recommended.
FRAX has limitations in that it doesn’t consider all the factors that increase risk, for example duration of diabetes or dose of oral steroids. For this reason a new calculator has been introduced called FRAXplus, which you can access at www.fraxplus.org. FRAXplus allows the score to be modified based on several additional risk factors.
There are still some risk factors not accounted for by FRAxplus, for example HIV, or long someone smoked, or the severity of a parent’s osteoporosis. And so it’s always important to use clinical judgment as well about risk.
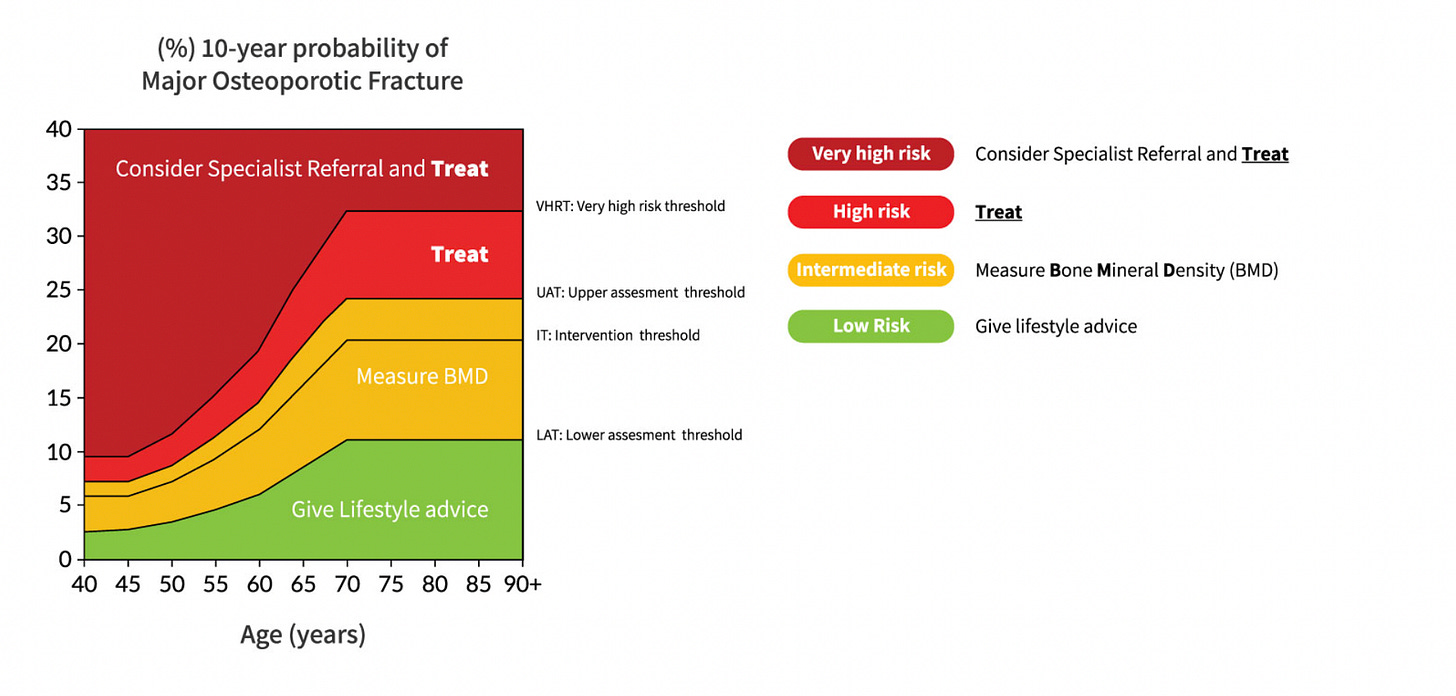
Also, there was a nice summary of when to decide on different therapies for osteoporosis or osteopenia from the National Osteoporosis Guideline Group in the UK. It’s important to know that guidelines vary country to country, as do FRAX scores, so this graph isn’t directly applicable outside of the UK, but it will be in the ballpark.
Lichen Sclerosus and Urine Exposure
This was completely new to me, but apparently there is a theory that urine exposure on the vulva is causative for lichen sclerosus (a skin condition that affects the vulva). This was more of an offhand comment in the Question and Answer section (if I remember correctly) than something covered in depth in the lecture. I did a little digging and apparently for men, irritation and inflammation from urine drops collecting beneath the foreskin is hypothesized to be a trigger for lichen sclerosus on the penis. Lichen sclerosus is apparently almost nonexistent in men circumcised at birth. For women, lichen sclerosus often first starts on or around the clitoral glans and hood, which is an area where urine droplets might collect. This hypothesis could explain why we see two age distributions for lichen sclerosus: younger children and then again starting around menopause, as skin changes from menopause may affect the skin barrier and the prevalence of incontinence rises. As chronic irritation and inflammation can disrupt the skin barrier, the idea that urine contact on the skin could play a role in the genesis of lichen sclerosus for women is a valid hypothesis.
I found one systematic review that didn’t find a link between lichen sclerosus and incontinence but a cross sectional study and a database review that did note a link between the two. Of course this is just a link, and may not be cause and effect because it’s also possible that women with lichen sclerosus may be more likely to have incontinence or dribbling because of scaring to their skin that impacts their urine stream. The large data base study found a more robust association between several autoimmune conditions and lichen sclerosus than incontinence and lichen sclerosus, but of course it could be two things–chronic irritation and inflammation from urine with a propensity for autoimmune conditions. A so-called “two-hit” hypothesis is common for many medical conditions.
More research is clearly needed, but the idea that women might want to consider preventatively moisturizing their vulva was discussed. I’ve been meaning to do a post on my favorite moisturizers and barrier ointments for the vulva, so this is a reminder for me to get going on that. Stay tuned!
Can Red Light Therapy Help the Menopausal Vulva?
This came up in the question and answer period. Apparently, women in online forums are recommending red light therapy for the vulva. This shocked the dermatologist and gynecologist who ran the session, and while this one is new for me, if people are asking about it online, I know from experience that there are some influencers who are already boasting about it for page views.
The answer? The dermatologist told us that that the benefit for red light therapy for the face is not robust, and it needs to be used every night for 30 minutes to get minor gains. Never mind, how it might actually be used? The conclusion was evidence for vulvar use is…lacking.
And there you have it. Day 1 at the 34th British Menopause Society Annual Scientific Conference is in the books, and it’s been a whirlwind of fascinating updates and thought-provoking talks. From the handy Edinburgh calcium calculator to the link between depression and the menopause transition and ending with a bit of a curveball: red light therapy for the vulva. It was also wonderful meeting many people whom I have come to know online.
There was some other content from Day 1 that I may want to dive into more deeply, so I’ll be working on that. Meanwhile, more from Day 2… coming soon!
References
Kirby, L., Gran, S., Kreuser-Genis, I., Owen, C. and Simpson, R. (2021), Is urinary incontinence associated with lichen sclerosus in females? A systematic review and meta-analysis. Skin Health Dis, 1: e13. https://doi.org/10.1002/ski2.13
Kirby, L., Gran, S., Orekoya, F., Owen, C. and Simpson, R. (2021), Is urinary incontinence associated with vulval lichen sclerosus in women? A cross-sectional study. Br J Dermatol, 185: 1063-1065. https://doi.org/10.1111/bjd.20583
Halonen P, Heikinheimo O, Hadkhale K, Gissler M, Pukkala E, Jakobsson M. Risk Factors for Lichen Sclerosus: A Case-Control Study of 43,000 Finnish Women. J Low Genit Tract Dis. 2024 Apr 1;28(2):164-168. doi: 10.1097/LGT.0000000000000796. Epub 2024 Jan 10. PMID: 38518214; PMCID: PMC11520338.





I wish you would investigate the use of drugs prescribed for osteoporosis. The anecdotal evidence of bad side effects (at least in my world) is far greater than what I've read about in the media (meaning I haven't scoured academic journals). A friend who'd undergone a long jaw surgery as a result of one of the drugs said that her doctor's waiting room was full of women who'd experienced similar side effects. Do you believe side effects are under-reported? I know many women (including a few physicians) who have decided not to take the drugs because of side effects as well as the effects on bone health after you must stop taking the drugs. I highly respect your thoughts on all health issues and would appreciate more on your thoughts about these drugs. Thank you!
In my personal experience, urine exacerbates LS, so at my clinic (Jean Hailes), the patients are encouraged to use Dermeeze ointment a few times a day to "coat" the mucosa on the vulva. My vulval dermatologist, Dr. Tanja Bohl, told me about a group of nurses who implemented this protocol at one particular aged care facility and reduced vulval dermatitis in patients by a significant amount. It's like urine is "corrosive". I don't have the right words, but to me, LS is about an impaired barrier and inflammation. If anyone is interested in learning about LS, etc, do a search on YouTube for "Dr Tanja Bohl." She is a fantastic educator. Love your work, as always. Enjoy the conference.